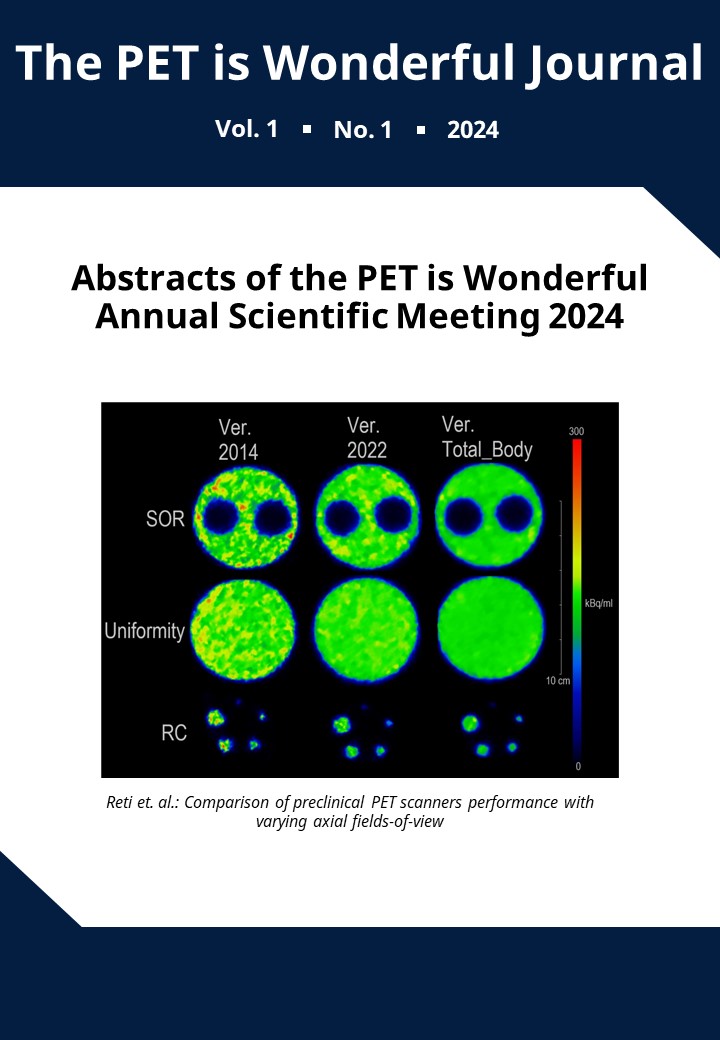
Comparing total-body metabolic PET imaging signatures of lung cancer cachexia to other wasting conditions
DOI:
https://doi.org/10.2218/piwjournal.9969Abstract
Cancer cachexia (CC) is a debilitating wasting condition. While anorexia and muscle wasting are features of cachexia, CC is a distinct and poorly understood metabolic syndrome1. We harnessed preclinical models of lung cancer to study how glucose uptake changes during CC and performed comparative analyses with anorexia and muscle wasting.
Lung K-rasG12D/+;Lkb1-/-(KL) mice suffering CC were imaged using [18F]fluorodeoxyglucose (FDG) PET at 15-19% weight loss alongside K-ras wild-type (WT) non-tumour bearing controls. Mice were injected with 12-18MBq of FDG, imaging 80-100 minutes post injection with the Mediso nanoScan® PET/MRI 1T.
In non-tumour bearing male animals, we modelled anorexia, muscle wasting, and circulation of cachexia factor GDF15 as follows, respectively: fasted overnight for 20 hr, treated with dexamethasone 21-phosphate (dexa) (2mg/kg, i.p. daily, 21 days) and single injection of recombinant human GDF15 hormone (0.1 mg/kg s.c.).
FDG ex vivo biodistribution showed increased uptake in myocardium (p<0.05), brain and liver (both p<0.01) of KL cachexic animals with no significant change in FDG blood pool.
Like cachexic KL animals, FDG uptake in fasted animals increased in brain (p<0.0001), perhaps due to increased FDG blood pool. FDG uptake in skeletal muscles and brown adipose tissue decreased (both p<0.05) in fasted state suggesting lower muscle activity and decreased thermogenesis respectively. Dexa-induced muscle atrophy also resulted in decreased FDG uptake in gastrocnemius/soleus muscles (SUVmean 0.63 vehicle vs. 0.45 dexa, p<0.05) but no changes in skeletal muscle FDG uptake were noted in cachexic animals. In contrast to KL animals, GDF15 injection resulted in decreased FDG uptake in myocardium (p<0.05).
Overall, the cachexic FDG signature showed differences to other wasting conditions in this mouse model, suggesting different underlying mechanisms.
Total-body PET can reveal diverse metabolic wasting states. We aim to elucidate mechanisms of metabolic changes in cachexia to allow metabolic subtyping and personalised treatment options.
Please click on the 'PDF' for the full abstract!
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Emma Brown, Lisa Duff, Federico Bernuzzi, Robert Bielik, Abdullah Alyamani, Chrysoula Vraka, Nesibe Peker, Fraser Edgar, Dmitry Soloviev, Johan Vande Voorde, David Lewis

This work is licensed under a Creative Commons Attribution 4.0 International License.