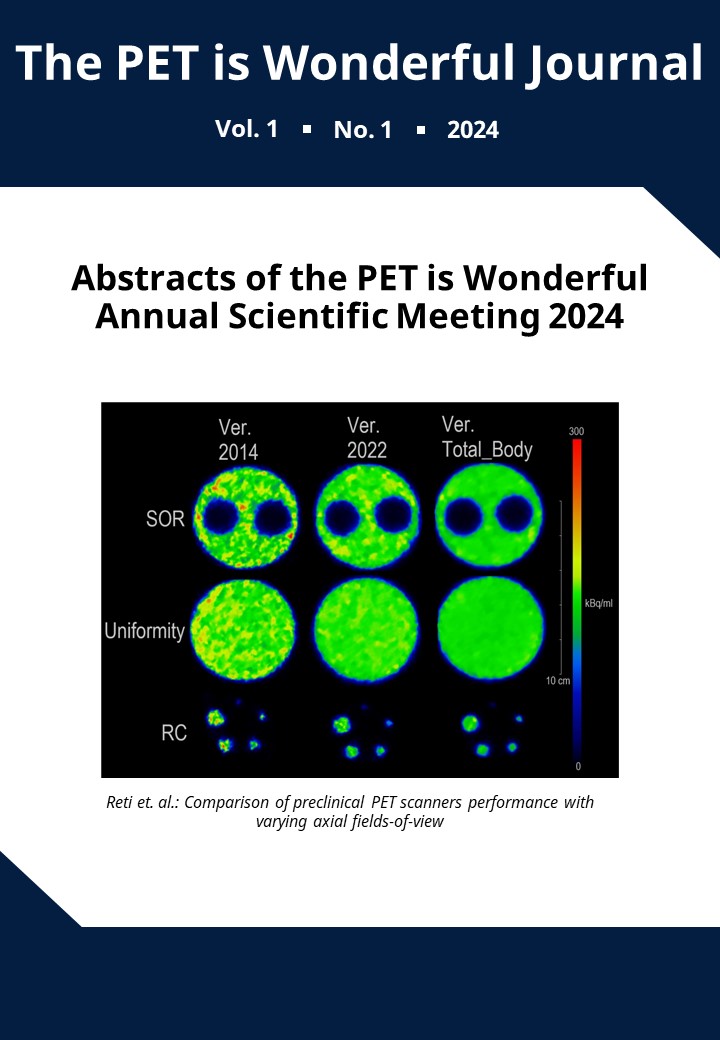
An instantly applicable machine learning model for extraprostatic extension prediction in prostate cancer: A post-hoc analysis of the RAPID 68Ga-PSMA PET/MRI trial
DOI:
https://doi.org/10.2218/piwjournal.9822Abstract
Radical prostatectomy (RP) is a common first-line treatment for patients with localized prostate cancer (PCa), with nerve-sparing techniques recommended to minimize adverse effects on quality of life. Accurate pre-operative detection of extraprostatic extension (EPE) remains difficult, often resulting in suboptimal treatment choices. Machine learning (ML) has the potential to improve pre-operative EPE detection through the integration of multimodal data, but its clinical adoption is hindered by implementation complexities and explainability issues.
This study conducted a post-hoc analysis of a prospective clinical trial (NCT02659527) involving patients who underwent [68Ga]Ga-PSMA-11 PET/MRI from 2014 to 2019. Only the clinically used parameters were collected, including PET-derived features, blood-based markers, histology-derived parameters, and patient demographics. ML models were developed using either only non-invasive features or a combination of non-invasive and invasive features for EPE detection and were compared to conventional PET image readings.
Among the 77 patients included in the study, 44 (57%) had EPE based on post-surgical findings. The ML models outperformed traditional PET image readings in diagnostic accuracy, with the explainable boosting machine (EBM) model achieving an AUC of 0.88. The ML model incorporating invasive features showed superior predictive ability for EPE compared to visual clinical assessments (AUC 0.88 vs. 0.71, p 0.02), while the difference between the ML model with non-invasive features and clinical assessments was not significant (AUC 0.83 vs. 0.71, p 0.34).
Explainable ML models utilizing routinely acquired clinical data can greatly enhance the pre-operative detection of EPE in PCa patients, potentially leading to more accurate clinical staging, better treatment decisions, and improved patient outcomes.
Please click on the 'PDF' for the full abstract!
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Jing Ning, Clemens Spielvogel, Kilian Kluge, David Haberl, Gabriel Wasinger, Laszlo Papp, Bernhard Grubmüller, Shahrokh Shariat, Lukas Kenner, Marcus Hacker, Alexander Haug, Sazan Rasul

This work is licensed under a Creative Commons Attribution 4.0 International License.