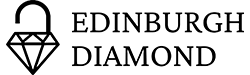Evaluating the potential of cis- and trans-4-[18F]fluoro-L-proline positron emission tomography as biomarkers of active collagen biosynthesis in cardiometabolic diseases
DOI:
https://doi.org/10.2218/piwjournal.10873Abstract
The increased prevalence of obesity and its associated comorbidities has coincided with an upsurge in cardiometabolic diseases, such as heart failure with preserved ejection fraction, metabolic dysfunction-associated steatotic liver disease, and chronic kidney disease. Active tissue remodelling and fibrosis following cellular damage and inflammation, characterised by aberrant collagen deposition, is a common feature of cardiometabolic disease. Currently, there are no established probes for non-invasive whole-body imaging of active collagen biosynthesis to study the multisystem consequences of cardiometabolic diseases.
We aimed to evaluate the potential of cis¬- and trans-4-[18F]fluoro-L-proline positron emission tomography (PET) as biomarkers of active misfolded and stable triple helical collagen biosynthesis, respectively, using a preclinical Western-style diet (WD)-induced rat model of cardiometabolic diseases.
Animals fed a WD had significantly greater uptake of both cis¬- and trans-4-[18F]fluoro-L-proline in the heart compared to age-matched controls across the time course, reflecting the increased histological accumulation of collagen, a profibrotic gene expression profile, and cardiac dysfunction on echocardiography. Although histological and transcriptional alterations were also noted in the liver, there were no detectable differences in hepatic cis- and trans-4-[18F]fluoro-L-proline PET signal. We hypothesise that the organ-specific differences in relative [18F]fluoro-L-proline PET uptake reflect disease-associated perturbations to the free-proline pool. Previous reports showed that WD feeding markedly increased the proline content of plasma and liver tissue1 which could in turn alter tracer kinetics. Currently, we are developing and validating new quantification strategies for [18F]fluoro-L-proline PET studies based on amino acid blood concentrations, similar to established plasma glucose corrections in [18F]fluorodeoxyglucose PET studies2, in order to improve [18F]fluoro-L-proline PET outcome reporting.
Overall, our data suggests that the rates of active collagen biosynthesis in cardiometabolic diseases are organ-specific, likely indicating differences in susceptibility to injury and fibrosis.
Please click on the 'PDF' for the full abstract!
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Angus Jacobs, Victoria J. M. Reid, Carlos-Alcaide Corral, Islay Cranston, Callum Sutherland, Heeyoun Hur, Adrian Thomson, Timothy J. Kendall, Timaeus E. F. Morgan, Kerry M. O’Rourke, Leanne M. Riley, Andrew Sutherland, Mark G. Macaskill, David E. Newby, Laura Denby, Jonathan A. Fallowfield, Adriana A. S. Tavares

This work is licensed under a Creative Commons Attribution 4.0 International License.